NTI update on PANDAS/PANS clinical trial
Our biotech Investment, Neurotech International (ASX: NTI) just put out an update on its Phase I/II PANDAS/PANS clinical trial.
For context - The initial trial was designed to run for ~12 weeks. After the initial 12 weeks all of the patients diagnosed with PANDAS/PANS were showing improvements and opted to prolong their treatments into an extension phase for another full year.
At the moment, NTI has all 15 of the patients initially recruited for the trial continuing on a 54-week extension phase for the trial.
Today, NTI put out data from the 24 week mark of the trial, here are our key takeaways from today’s announcement:
- Strong safety/tolerability.
NTI confirmed that between the 12 and 24 week mark of the trial patients have shown “no adverse or serious adverse events”.
That means that since inception NTI’s treatment has shown close to no serious adverse side effects for patients who are using NTI164.
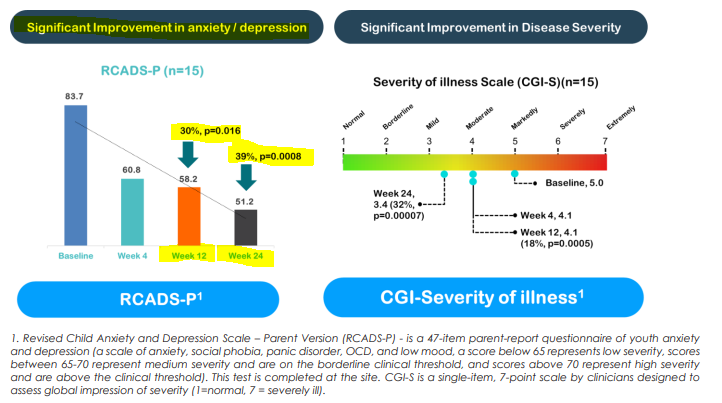
- Endpoint for anxiety/depression
NTI met the endpoint for anxiety and depression “with a 30% improvement in overall symptoms from high severity at baseline to low severity from week 4 onwards”.
NTI also showed a 39% improvement by week 24.
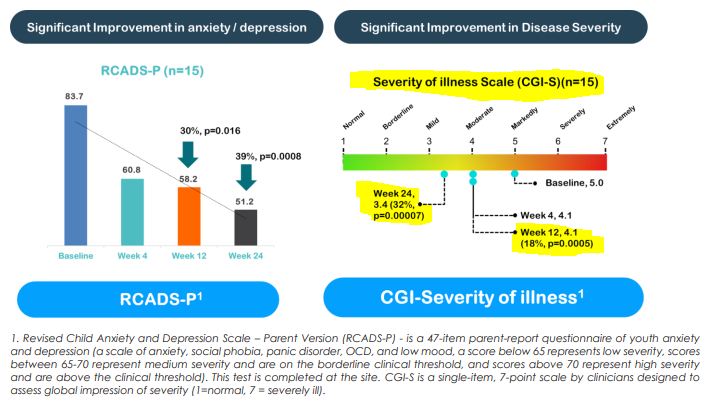
- Endpoint for severity of illness
NTI also showed an improvement in the severity of illness by ~18% at the 12 week mark and 32% at the 24 week mark.
- NTI to start regulatory discussions
NTI also confirmed that it would start early discussions with regulators around progressing the development of its treatment for PANDAS/PANS.
At the moment there are no approved treatments for PANDAS/PANS, which is an orphan disease meaning any treatments could be eligible for “orphan drug designation”.
NTI has previously mentioned it is looking to get Orphan Drug Designations for its NTI164 treatment for PANDAS/PANS in the US and EU.
Orphan drug designation is important because it often means the proposed treatment is eligible for:
- Fast Tracked Approvals
- Use of Surrogate Endpoints
- Market exclusivity
- Lack of competition
- High unmet medical needs
🎓To learn more about orphan drugs check out our educational article here: Orphan Drugs Explained
What is PANDAS/PANS?
PANDAS (Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections) and PANS (Paediatric Acute-onset Neuropsychiatric Syndrome) are conditions that affect children, resulting in sudden changes in behaviour and mood.
PANDAS is specifically linked to strep infections, however PANS can be caused by various infections.
- Causes: PANDAS is thought to be triggered by strep infections in children. Whereas, PANS can be set off by various infections, toxins, and other triggers. Both disorders are autoimmune in nature, where the child’s own cells mistakenly attack healthy brain cells.
- Symptoms: Sudden onset of OCD, tics, anxiety, emotional lability and some motor abnormalities. Symptoms may worsen acutely.
- Prevalence: Research suggests that PANDAS impacts 1 in every 200 children, however prevalence of PANS is less clear.
- Treatments: There is no simple treatment for PANDAS/PANS. Treatment usually involves antibiotics, anti-inflammatories and immunotherapy.
What’s next for NTI?
Phase I/II Rett Syndrome clinical trial (results in Q1 CY2024) 🔄
We are hoping NTI can show that its treatment is safer and has similiar (we hope better) efficacy to $2.6BN capped Neuren’s Daybue treatment.
Especially considering the recent short report drama, any positive news could re-rate NTI’s share price to the upside.
Phase II/III trial for Autism Spectrum Disorder (results in Q1/Early Q2 2024) 🔄
After promising Phase I/II trial results, we see this trial as important from a commercialisation perspective.






