NTI adds impressive board member
Hot off the heels of releasing strong positive data from two clinical trials for Autism Spectrum Disorder (ASD) and Rett Syndrome (results were good enough to seal a quickfire $10M raise) - our biotech Investment Neurotech International (ASX: NTI) has appointed a new board member with serious pedigree.
The latest NTI announcement details the appointment of Robert Maxwell Johnston as a Non-Executive Director.
Welcome to NTI Mr Johnston:

Johnston brings significant relevant experience - having been President and Chief Executive officer of Johnson and Johnson Pacific for 11 years.
J&J is capped at $546BN and is the world's largest healthcare provider.
We think this appointment by NTI signals a new phase for the company that will be increasingly focussed on potential commercialisation and licensing opportunities.
In other words, potential deals to bring NTI’s biopharmaceutical to market.
Johnston’s commercial acumen and industry connections should certainly help.
Here’s a quick snapshot of Johnston’s current or recent positions with ASX-listed companies:

Experience with Polynovo brings an understanding of innovative medical products, Probiotec is a medicine manufacturer - so plenty of operational knowledge, and AusCann is a cannabis company so Johnston understands cannabinoids.
In short, we think the perfect appointment for NTI.
We place special emphasis on board quality in our due diligence process - and it's hard to understate the importance of good management and leadership in driving positive outcomes for the companies we Invest in.
We’re looking forward to seeing what Johnston can bring to the role in this new phase for NTI - here’s what is coming next…
What’s next for NTI?
There’s a heap of newsflow to come, we’ve highlighted potential catalysts below:
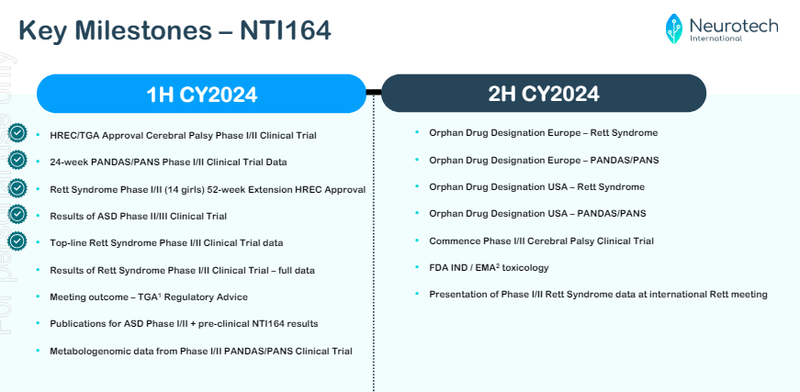
(Source)
Here’s our highlights of what’s next for NTI for the rest of year with our commentary:
Results of Rett Syndrome Phase I/II Clinical Trial - full data
This will include results from NTI’s secondary endpoints - and paint a better picture of its potential efficacy across these metrics.
[Potential catalyst] Meeting outcome - TGA Regulatory Advice
Given the ASD results, we expect NTI will engage with the Therapeutic Goods Administration (TGA) in Australia to see if the TGA supports the treatment entering the market.
Metabologenomic data from Phase I/II PANDAS/PANS Clinical Trial
This is actually quite important - this is another measure of how well NTI’s treatment works for another rare paediatric disorder called PANDAS/PANS.
Results have already come back positive for this disorder in October of last year - this could show the method of action of NTI’s treatment, further strengthening its case for entry to market.
Read about PANDAS/PANS:
NTI’s clinical trial meets primary endpoints for neurological disease
[Potential catalyst] Orphan Drug Designation Europe - Rett Syndrome
Orphan Drug Designations are very valuable and can fetch large sums of money - Neuren’s Orphan Drug Designation was worth roughly $100M. If the EU gives the all clear, this catalyst could re-rate NTI’s share price.
🎓Learn: Orphan Drugs Explained
[Potential catalyst] Orphan Drug Designation Europe - PANDAS/PANS
Same applies for PANDAS/PANS
[Potential catalyst] Orphan Drug Designation USA - Rett Syndrome
The US healthcare market is incredibly lucrative - US Orphan Drug Designation for Neuren played a major role in its 2000% re-rate.
[Potential catalyst] Orphan Drug Designation USA - PANDAS/PANS
Same applies for PANDAS/PANS in the US
Completion of Patient Recruitment Phase I/II Cerebral Palsy
We expect NTI to start recruiting for this trial which could add another big string to NTI’s bow.
NTI already has Human Research Ethics Committee (HREC) approval and Clinical Trial Notification (CTN) scheme clearance by the Therapeutic Goods Administration (TGA) for this trial.
Cerebral Palsy is a group of permanent movement neurological disorders, appearing in early childhood.
It impacts muscle coordination and motor-neuron skills, but can often impact other body parts too. No two people experience CP in the same way.
Market opportunity: An annual drug therapy market of US$4.3BN.
Rare disorder - potential orphan drug designation could make NTI’s treatments lucrative.
At the moment there are only two FDA approved drugs available.
Commence Phase I/II Cerebral Palsy Trial
Starting the trial would open up another catalyst for NTI on results.
FDA IND/EMA toxicology
US FDA Investigational New Drug and European Medicines Agency toxicology reports will hopefully show that NTI’s treatment is safe in the human body - this can only strengthen NTI’s case for market entry.
Presentation of Phase I/II Rett Syndrome data at international Rett Syndrome conference
Neuren previously presented its results here - we’d like to see NTI do the same to build support for its treatment in the broader Rett Syndrome community.






